Service Regulation in Colombia
The Passarini Group helps your company to obtain your product registration before the INVIMA (Colombia’s National Food and Drug Surveillance Institute), regulator body of themedical products market in the country, and for this, counts with the support of its consultants specialized in record of any type of product.
According to the Colombia legislation, to a product be commercialized in its internal market, it is necessary to prove that the same is registered in their origin country, in accordance with the GHTF (the Global Harmonization Task Force).
The classification of the product follows the rules determined by the health legislation. The Passarini Group counts with a specialized team, qualified and able to provide all the necessary support to obtain your product registration in the Colombian market. Meet Service Regulation in Colombia.
Quality System
Record Holder
Regulatory Consulting
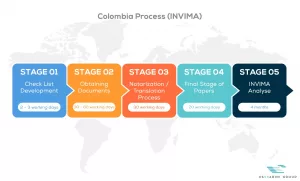
See below our complete INVIMA process flow
Passarini Group is a company that has the best specialists in Regulatory Consultancy of Medical Products, applying the One Stop Shop concept daily in your company with practical solutions.




