Service Regulation in Canada
Canada, with its population of over 38 million and a well-established national healthcare system, offers excellent market opportunities for medical device manufacturers.
To market your medical device in Canada, it is necessary to comply with the Medical Device Single Audit Program (MDSAP), which covers compliance with the Canadian Medical Device Regulations (CMDR) quality management system (QMS) requirements and obtaining the necessary approval from Health Canada.
Health Canada Medical Device Licensing
In Canada, a Medical Device Establishment License (MDEL) is required for marketing Class I medical devices, and a Medical Device License (MDL) is required for Class II, III, or IV devices.
If your intention is to distribute licensed medical devices in Canada, an MDEL will be required.
Health Canada’s Quality System Requirements for Medical Devices and ISO 13485
Health Canada mandates that Class II to IV medical device manufacturers comply with the ISO 13485 Quality Management System (QMS) requirements under the MDSAP, ensuring compliance with the CMDR.
Prior to marketing your device in Canada, your compatible QMS must undergo an audit conducted by a Medical Device Single Audit Program (MDSAP)-accredited Auditing Organization (AO).
See below our complete Health Canada process flow
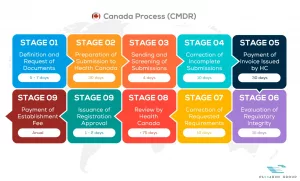
Let us assist you with ISO 13485 and MDSAP Regulatory Compliance
Passarini Group has highly experienced consultants who specialize in helping medical device manufacturers, suppliers, or importers achieve compliance with MDSAP requirements, enabling them to gain access to the Canadian market.
Our experienced consultants have provided assistance to a large number of medical device companies, guiding them through the licensing process with Health Canada, ensuring compliance with MDSAP and CMDR requirements, in addition to applying the One Stop Shop concept daily in your company with practical solutions.



