Service Regulation in Chile
The current Chilean regulation for medical devices focuses its work on the National Agency for Medical Devices, Innovation and Development (ANDID), one of the departments of the Chilean Institute of Public Health (ISPCH). The ANDID agency was born in November 2020, with one of its central objectives being to ensure the safety and performance of medical devices used in the country.
The current regulatory model establishes that there are 9 medical devices for which health registration with the ISPCH is required, which will allow their distribution, commercialization and/or use.
We offer the following services for medical devices in Chile:
Single-use Latex Surgical Gloves: Exempt from Decree No. 342/2004
Latex gloves for medical examination: Exempt from Decree No. 342/2004
Condoms – Rubber latex condoms: Exempt from Decree No. 342/2004
Sterile single-use hypodermic needles: Exempt from Decree No. 1887/2007
Single-use sterile hypodermic syringes: Exempt from Decree No. 1887/2007
Condoms – Synthetic Male Condoms: Exempt from Decree No. 93/2018
Condoms – Female Condoms: Exempt from Decree No. 93/2018
Portable Automated External Defibrillators (AEDs): Exempt from Decree No. 42/2021
In vitro Diagnostic Medical Devices for HIV detection: Exempt from Decree No. 41/2022 (entry into force from December 16, 2022)
Each of these medical devices has been incorporated into the health control regime in accordance with the provisions of the indicated exempt decrees, which also provide information on their risk classification. For their registration, each one must comply with the compliance verification, in which quality parameters are ensured that allow the commercialization of safe and effective products.
Current national regulations establish that, in order to obtain the sanitary registration of the MD, a conformity verification certification must be previously obtained, granted by an entity accredited by the National Institute of Standardization (INN) and authorized by the ISP.
Our team offers a wide range of services in Regulatory Affairs
Medical devices not contemplated in said exempt ordinances are not subject to a sanitary control regime, for which they do not require sanitary registration to be marketed.
This category includes Implantable Medical Devices, Single Use Medical Devices, Medical Equipment, Medical Devices for in vitro diagnostics, Reusable Medical Devices, Medical Devices for surgical use, Medical Devices for ophthalmological use, Medical Devices for orthopedic use and others.
In order to be able to market Medical Devices in Chile, there are different procedures in the case of DM that require sanitary registration and those that do not, understanding that those subject to a sanitary control regime imply greater inspection in their processes.
Considering the existence of a significant number of medical devices sold in Chile, the existence of a surveillance system that provides information to those involved in the use of medical devices is essential. Technovigilance aims to improve health protection, both for patients and users, reducing the likelihood of adverse events occurring in clinical practice.
Technical Standard No. 204, On Patient Safety and Quality of Care in the Safety of Using Medical Devices: Technovigilance corresponds to the essential regulatory framework in this matter. This standard represents a major step forward in strengthening medical device regulation, specifically at the post-marketing surveillance stage.
Today in the national congress (Senate) the new modification of the current law of medicines and supplies (Law 20.724) is discussed, in this article Medical Devices makes a very special mention to normalize its regulation in Chile, but without mentioning the increase of products subject to regulation.
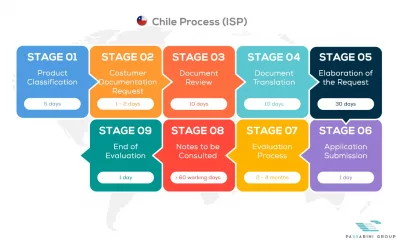
See below our complete ISPCH process flow
Passarini Group is a company that has the best specialists in Regulatory Consultancy of Medical Products, applying the One Stop Shop concept daily in your company with practical solutions.




