Service Regulation in Bolivia
We advise and accompany you to obtain the importation of pharmaceutical and medical products. If you are thinking about importing, manufacturing medicines, hygiene and cleaning products, cosmetics, or medical devices, we want to know you!
We offer advisory services, specialists in Business Opening Procedures, Registration of Pharmaceutical Products (Sanitary Registration of Medications, Reagents, Medical Devices, Sanitary Notifications of Cosmetics and Cleaning Products).
In addition to obtaining different certifications, Customs Clearance, Current Business Certificate, Good Storage Practices Certificates, Regular Inspection Certificates issued by the Regulatory Entity of Bolivia AGEMED (State Agency for Medicines and Health Technology), we have trained and technologically updated personnel using a modern system that facilitates and streamlines our processes.
Our team offers a wide range of Regulatory Affairs services:
Ongoing advice to importing companies and pharmaceutical laboratories in accordance with current regulations issued by the regulatory body
Advice for expansion and relocation of warehouses and companies
Advice alterations, modifications or expansion of the information declared at the beginning of the activities of the different companies
Management of procedures before regulatory authorities AGEMED
Understand the details of the main services:
- Assistance in obtaining documentation to comply with the requirements for opening importers requested by the regulatory body;
- Advice on compliance with Good Storage Practices (GSP) for approval of facilities in the process of opening a company;
- Guidance on the storage, distribution and transport of medicines, medical devices, and other products;
- Guidance on the sanitary registration or mandatory health notification of pharmaceuticals products, medical devices, or other products.
- Management of sanitary registrations of medicines, natural medicines, biological products, galenic products, dietetic and sweeteners, medical devices and biomedical equipment (classes I, II, III and IV) and reagents;
- Dossier follow-up;
- Sanitary registration changes management;
- Updating inserts, technical sheets, labels, etc;
- Technical translation English-Spanish, Portuguese-Spanish. Pharmaceutical technical documentation;
- Preparation of responses to notification letters;
- Qualification before the CFN (National Pharmacological Commission) of new molecules.
Registration of medical devices is the authorization given to a manufacturer, importer, or trader to distribute their product in the bolivian market, after complying with all the requirements established in current regulations.
Medical device is any instrument, device, implement, machine, artifact, material or other similar or related article, used alone or in combination, including accessories and software necessary for its correct application proposed by the manufacturer in its use with human.
Beings for diagnosis, prevention, monitoring, treatment or alleviation of disease or injury, replacement or support of an anatomical structure or physiological process, birth control, disinfection of medical devices, in vitro examination of samples derived from the human body and which do not meet their intended basic action in human body by pharmacological, immunological or metabolic means, but who can be aided in their functions by such means.
See below our complete AGEMED process flow
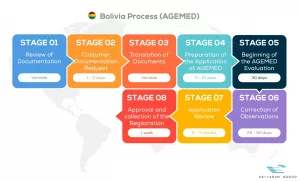
Passarini Group is a company that has the best specialists in Regulatory Consultancy of Medical Products, applying the One Stop Shop concept daily in your company with practical solutions.
- Local representation for foreign manufacturers or distributors of pharmaceuticals, medical devices, and others;
- Submission to the regulatory body (AGEMED) of information and technical documentation relating to the products for their evaluation and approval;
- Custody of technical dossier;
- We have the Certification in Good Storage Practices by AGEMED.
- Advice on alterations, modifications, or expansion of the information declared at the beginning of the activities of the different companies;
- Management of procedures before regulatory authorities AGEMED;
- Procedure for submitting drug advertising to the Pharmacological Commission in accordance with current regulations;
- Advice on trademarks and patents;
- Management of trademark and patent processes at SENAPI;
- Management procedures for food approval at SENASAG;
- Management of import processes for clothing, footwear, furniture, and others at SENABEX.
- Management of sanitary notifications (NSO) of cosmetic products, domestic hygiene products, personal hygiene absorbent products;
- NOS recognition of health products from member countries of the Andean Community (CAN);
- NSO renewal;
- Elaboration of arts, technical sheets, labels, etc;
- Dossiers follow-up;
- Preparation of responses to notification letters;
- Management of changes in sanitary notification.




