Regulation of Services in Argentina
Manufacturers of medical products wishing to register their products in Argentina must comply with the requirements of the National Administration of Medicines, Foods and Medical Technology – ANMAT.
Medical products must be registered by a company located in Argentina and authorized by ANMAT as Importer of Medical Products. According to Argentine regulations, it is preferable for registration that the manufacturer has a Free Sale Certificate from a country with high sanitary surveillance. Otherwise, ANMAT must inspect the manufacturer’s plant at the source.
We offer the following services in Argentina:
The registration includes the preparation, submission and follow-up of the files to be sent to ANMAT and their technical evaluation. Response to all ANMAT’s necessary demands. If necessary, translations can be included.
Hosting of registration in our associated company authorized by ANMAT (Registration Holder)
Maintenance and annual updating of registration in accordance with the manufacturer’s requirements.
Audits at commercial distributors meeting the specifications of the product manufacturer, audits of Good Manufacturing and Distribution Practices or audits in accordance with ISO 13485.
Advice on regulatory issues for medical products that can be marketed in Argentina.
Investigation of competing products, as well as verification of adverse events and recalls.
Advice on carrying out tests on medical products, as well as preparing validation reports.
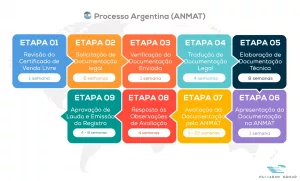
See below our complete ANMAT process flow
Passarini Group is a company that has the best specialists in Regulatory Consultancy of Medical Products, applying the One Stop Shop concept daily in your company with practical solutions.




