Medical device regulation in South Africa
The regulation of medical devices in South Africa is carried out by the South African Health Products Regulatory Authority (SAHPRA). This government agency is responsible for ensuring that all medical devices marketed in the country are safe, effective and of high quality.
The regulatory process involves a detailed assessment of each device, taking into account factors such as its composition, functionality, associated risks and clinical efficacy. Medical devices are classified into categories, depending on the degree of risk they present to patients and users.
Check out some of the services we provide in South Africa:
Classification Recommendation
Medical Device Compliance Review
License Amendment (Hosting) or License Application
Amendment (Hosting) or Submission of Application
Importation Administration
Distribution Administration
1. Classification Recommendation
The starting point for all medical devices and IVDs intended for the South African
market undergo a formal classification and compliance review according to the local
guidelines, following the receipt of the following technical documentation:
Your Content Goes Here
Your Content Goes Here
Your Content Goes Here
- MHRA UK
- Japan PMDA
- ANVISA
- Health Canada
- Australian TGA
- US FDA PMA
- WHO
Your Content Goes Here
The medical device regulatory framework has a classification system for medical
devices and IVDs, as per the medical device Regulations of Act 101 of 1965 set out
in the Classification Rules of Medical Devices. The manufacturers intended us of the
medical device is crucial when determining the classification of a medical device.
Medical devices are classified in the following levels:
| Classification | Level of risk |
|---|---|
| Class A (Class I) | Low risk |
| Class B (Class IIa) | Low – moderate risk |
| Class C (Class IIb) | Moderate – high risk |
| Class D (Class III) | High risk – where risk relates to the patient or to the public health |
The outcome of the classification and compliance review will be a Classification
Recommendation document and a Medical Device Compliance Review document.
The Classification Recommendation provides details specific to the product and the
classification rules best fit to the product. The Medical Device Compliance Review is
an analysis of the provided label and IFU artwork against the Essential Principles
guideline, resulting in a document indicating any changes to be made on either the
label or IFU as necessary.
2. Medical Device Compliance Review
Following the completion of the classification process, the medical devices and IVDs
are assessed according the Essential Principles of Safety and Performance of
medical devices and IVDs. This guideline provides the requirements relating to the
safety and performance characteristics of medical devices and IVDs.
It is the responsibility for the license holder to demonstrate compliance for their
medical devices and IVDs withing South Africa.
There are six general Essential Principles of Safety and Performance that apply to all
medical devices. There are a further nine Essential Principles of Safety and
Performance about design and construction to apply to devices on a case-by-case
basis.
- Use of medical devices not to compromise health and safety
- Design and construction of medical devices to conform to safety principles
- Medical devices to be suitable for intended purpose
- Long term safety
- Medical devices not to be adversely affected by transport or storage
- Benefits of medical devices outweigh any side effects
- Chemical, physical and biological properties
- Infection and microbial contamination
- Construction and environmental properties
- Medical devices with a measuring function
- Protection against radiation
- Medical devices connected to or equipped with an energy source
- Information to be provided with medical devices
- Clinical evidence
- Principles applying to IVD medical devices only
Manufacturers can demonstrate that the Essential Principles of Safety and
Performance have been met for a medical devices, in the following ways:
- A documented and detailed risk analysis
- The results of testing of the medical device
- Literature searches
- Copies of the label, packaging and Instructions for Use to demonstrate that
information requirements have been met - Expert opinion
- The design dossier, if applicable
The information must be held by the license holder and provided to the Authority
upon request.
Standards that are commonly used by the medical device manufacturer are:
- ISO 14971 – Application of risk management to medical devices
- ISO 13485 – Quality Management Systems: Requirements for regulatory
purposes - ISO 10993 – Biological evaluation of medical devices
3. License Amendment (Hosting) or License Application
The final step to entering the South African market is to acquire licensing of the
products. There are two main ways of achieving this, through Passarini Group’s
hosting services, or through the application of a medical device license.
Upon evaluation of the intended outcome, Passarini Group will advise on the most
applicable application, and provide the necessary services for submission.
A medical device manufacturer’s license is a regulatory authorisation issued by
SAHPRA granting permission to a company or organisation to manufacture and
distribute medical devices in South Africa.
To obtain a manufacturer’s license, the
company must demonstrate compliance with the regulatory requirements and
standards set forth by the regulatory authority. This includes adherence to quality
management systems (ISO13485:2016), product safety and performance principles,
and other applicable regulations specific to medical devices.
The manufacturer’s license includes provisions for the applicant to include import,
export and distribution activities, however it does not permit the purchasing of
locally manufactured or distributed product. The documents included in the
submission include the Quality Manual, SOP Index and product technical
documentation.
Similar to the license to manufacture, import, export or distribute, the wholesaler’s
license is a regulatory authorisation issued by SAHPRA granting permission to a
company or organisation to purchase locally sourced medical devices.
To obtain a wholesaler’s license, the company must demonstrate compliance with the regulatory requirements and standards set forth by the regulatory authority. This includes
adherence to quality management systems (ISO13485:2016), product safety and
performance principles, and other applicable regulations specific to medical devices.
The wholesaler’s license includes provisions for the applicant to include wholesaling
and distribution activities, however it does not permit the manufacturing, importation or exportation of product. The documents included in the submission include the Site Master File, SOP Index and product technical documentation.
4. Hosting Services
Passarini Group holds a license to import, export and distribute medical devices and
IVDs of all classes, enabling the provision of hosting services to clients wishing to enter the South African market with confidence.
The company operates on an ISO13485:2016 quality management system, with implemented procedures to ensure compliance to all applicable regulations for your product. In addition to this, Passarini Group provides regulatory representation to the SAHPRA ensuring protection to uncertainties.
Process conducted include final product release control and responsibility, adverse device reaction reporting, product recalls, importation
administration and distribution administration. Through the hosting service, your company is able to enter the South African market with confidence in compliance.
5. Import Administration
As a requirement for all medical devices and IVDs being imported into South Africa, only a licensed entity is permitted to conduct these activities. Understanding the complexities and uncertainties of the importation process, Passarini Group provides services to ensure product enters the market with streamline precision.
As a hosting client of Passarini Group, this service is included and mandatory, however it is an additional service for all clients.
6. Distribution Administration
As a requirement for all medical devices and IVDs being imported into South Africa, only a licensed entity is permitted to conduct these activities.
Understanding the complexities and uncertainties of the importation process, Passarini Group provides services to ensure product enters the market with streamline precision.
As a hosting client of Passarini Group, this service is included and mandatory, however it is an additional service for all clients.
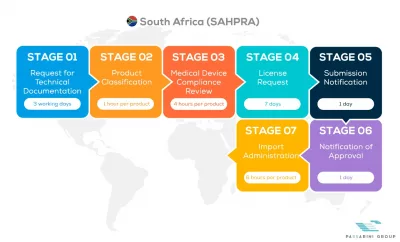
See below our complete SAHPRA process flow
Let us assist you with SAHPRA Regulatory Compliance
Passarini Group has highly experienced consultants who specialize in helping medical device manufacturers, suppliers, or importers achieve compliance with SAHPRA requirements, enabling them to gain access to the African market.
Our experienced consultants have provided assistance to a large number of medical device companies, guiding them through the licensing process, while also implementing the One Stop Shop concept in your company with practical solutions on a daily basis.




