Regulation in Ecuador and more Services
Manufacturers of medical devices (dental products, biomedical equipment, biochemical and diagnostic reagents) who wish to distribute and market their products in Ecuador must comply with Ecuadorian legislation, that is, for a medical device to enter the country, it must obtain the health registration issued by the health authority (National Agency for Sanitary Regulation, Control and Surveillance – ARCSA), which is a unit of the Ministry of Public Health (MSP).
Passarini Group, through its technical and legal team in Ecuador, offers the comprehensive advice and support you need to comply with the regulatory framework in all its stages.
In Ecuador we have the following services:
The sanitary Registration is the certification granted by the National Health Authority, through ARCSA, for the manufacture, assembly, import, distribution, and commercialization of medical devices for human use.
In Ecuador, we help companies prevent, resolve compliance issues, and develop effective strategies for submitting and approving health registrations.
Classes of product that we regularize:
- Food and Nutritional Supplements
- Cosmetics
- Medical Devices, Dental Products
- Biochemical and IVD reagents
- Medicines
- Natural products for medicinal use
- Pesticides for domestic, industrial, and public health use
- Hygienic products for domestic and industrial use
As an alternative to creating a Branch in Ecuador, the hosting service for health registers is being considered. This service allows owners of products that require a health registration (authorization to market products in Ecuador) not to hand over health registration to distributors and keep them under their control.
Benefits:
- Foreign companies that own the product can establish business relationships with various local importers/distributors in Ecuador;
- Client can change, add, or delete local importers/distributors according to their commercial interest;
- If the client so decides, the Health Registration can be transferred at any time to another local applicant.
Passarini Group has state-of-the-art Consultants and Auditors able to implement Quality Management Systems, Good Storage, Distribution and Transport Practices, has already contributed to several manufacturers obtaining the necessary certifications.
This area includes the services of:
- Diagnostic audit to know the current state of the company;
- Advice on the implementation of Good Storage/Distribution and Transport Practices (BPADT).
As established by the regulation of storage, distribution, and transport of medical devices regarding facilities, equipment, procedures, organization, personnel and other, there establishments (warehouses) must guarantee the maintenance of characteristics and properties of the products during storage, distribution, and transport.
We provide the costumer with a certified warehouse service to store their products in appropriate conditions, complying with Ecuadorian legislation.
The integration of services has been a competitive advantage in the sector, that’s why we offer our clients all the regulatory and logistical services, so that their products reach the Ecuadorian market, complying with all the regulatory and customs framework.
Among the services we have:
- Coordination and monitoring of imports and exports;
- Foreign trade outsourcing;
- Search for international suppliers;
- International business advice.
Our area on the contractual protection of all your company’s information, relating to the people who intervene directly or indirectly in your business, through the protection of brands, plant varieties, copyright and advertising.
Services:
- Assistance with database updated since 1895;
- Phonetic search;
- Registration of trademarks and patents;
- Trademark renewal.
Technossurveillance is the set of activities aimed at identifying, collecting, evaluating, managing, and disclosing adverse events or incidents resulting from the use of medical devices for human use; as well as the identification of risk factors associated with them, to prevent their appearance and minimize their risks.
Among the services we offer are:
- Immediate notification of serious and non-serious adverse events or incidents to the National Technovigilance System of the Ministry of Public Health;
- Periodic notification of serious and non-serious adverse events or incidents to the National Technovigilance System of the Ministry of Public Health.
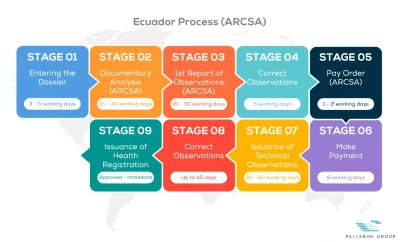
See below our complete ARCSA process flow
Passarini Group is a company that has the best specialists in Regulatory Consultancy of Medical Products, applying the One Stop Shop concept daily in your company with practical solutions.




