Medical Device Regulation in Russia
The Russian medical device market offers great potential for foreign companies. However, the process of registering and marketing medical products in Russia is complex and requires specialized knowledge.
Passarini Group, with its extensive experience in international regulation, offers a comprehensive set of services to help companies efficiently and safely access the Russian market.
The regulatory agency responsible for approving medical devices in Russia is Roszdravnadzor (RZN).
Roszdravnadzor is the Federal Service for Surveillance in Healthcare and Consumer Rights Protection of the Russian Federation. It is responsible for regulating a wide range of health-related activities, including the registration and control of drugs, medical products, and cosmetics.
The regulatory process involves a detailed evaluation of each device, considering factors such as composition, functionality, associated risks, and clinical effectiveness. Medical devices are classified into different categories, depending on the degree of risk they pose to patients and users.
Services offered by Passarini Group in Russia:
Medical Device Registration: Preparation and submission of technical dossiers, monitoring of the evaluation process and obtaining the registration license. We assist you throughout the entire process of registering your medical device with Roszdravnadzor, ensuring that all necessary documentation is prepared and submitted correctly.
Medical Device Classification: The classification of medical devices in Russia is a crucial step in the regulatory process. Our specialized team assists in determining the appropriate class for your product, based on the guidelines established by Roszdravnadzor.
Translation and Adaptation of Documentation: Translation and adaptation of labels, instructions for use, and other technical documents into Russian, required for the registration of your medical devices in Russia.
Legal Representation: Acting as the legal representative of the company before the Russian regulatory agency. We offer local representation services for foreign manufacturers, acting as an intermediary between your company and the Russian regulatory authorities.
Hosting Services: Passarini Group has a license to import, export, and distribute medical devices and IVDs of all classes, allowing us to provide hosting services for clients who wish to enter the Russian market with confidence. We operate under an ISO 13485:2016 quality management system, ensuring compliance with all applicable regulations.
Regulatory Consulting: Consulting on regulatory requirements, technical standards, and procedures. We provide comprehensive and specialized support for companies wishing to enter and operate in the Russian medical device market. Our experience and knowledge of local regulations ensure an efficient process that complies with all Roszdravnadzor requirements.
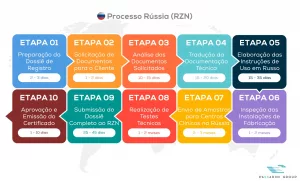
Simplified flowchart of the medical device registration process in Russia
Dossier Preparation: Translation of all technical documentation into Russian, preparation of instructions for use in Russian, and factory inspection.
Pre-registration Procedures: Importation of samples to Russia, conducting tests in local laboratories, and delivery of samples to clinical centers.
Local Tests: Conducting technical, clinical, biocompatibility, electromagnetic, and metrology tests, according to the device’s classification.
Dossier Finalization and Submission: Finalization of the dossier with the test results and submission to Roszdravnadzor.
Final Decision: Approval of registration and issuance of certificate.
See the full flowchart outlining the steps involved in the Russian registration process
Want to market your medical products in Russia?
Passarini Group offers a full range of services to assist you in this process.
For more detailed and specific information on medical device regulation in Russia, please contact us and schedule a preliminary consultation.
We have expertise in international regulation and can provide comprehensive support for the registration of your products in the Russian market.
Simplify your access to the Russian market! Send an email to contato@passarini.com.br




