Service Regulation in Paraguay
We are a company that provides regulatory affairs services to companies that manufacture Medical Devices, piercing-cutting materials and personal protective equipment classes I, II, III and IV, providing each client with the necessary information for registering products according to their classification before the National Directorate of Health Surveillance (DNVS).
To carry out all the work, we have a work team composed of trained professionals in the area of conducting.
Our team offers a wide range of Regulatory Affairs services:
Refers to the technical responsibility of the Pharmaceutical Chemist professional known as Regent within companies for their authorization from the National Directorate of Sanitary Surveillance in Health, for compliance with Good Manufacturing and Storage Practices, also for guidance advice regarding sanitary registration, in accordance with the regulations in force.
Establishment Authorization procedures, management of Sanitary Records, management of changes, other miscellaneous requests.
Elaboration of technical sheets according to the format established by DNVS for product registration, including the elaboration and review of packaging labeling in accordance with current regulations.
We offer advice on regulatory management to pharmaceutical establishments and other chemical professionals for starting activities, expanding items, transferring deposits, obtaining a Certificate of Good Practices, sanitary records, among others.
Translation services for technical documents by a private translator without registration and translation of legal documents signed by a translator registered with the Supreme Court of Justice of Paraguay. Ex.: Inglês – Espanhol/Espanhol-Inglês; Português-Espanhol/Espanhol-Português.
For foreign companies that do not have a presence in Paraguay and wish to enter the local market, we offer local regulatory representation to regularize product registrations in Paraguayan territory.
In this way, they can start marketing the products through local distributors without having to wait for the entire authorization process, management of good storage practices and other processes to be approved.
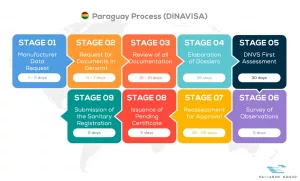
See below our complete DINAVISA process flow
Passarini Group is a company that has the best specialists in Regulatory Consultancy of Medical Products, applying the One Stop Shop concept daily in your company with practical solutions.




