Regulation of Services in Argentina
Manufacturers of medical products wishing to register their products in Argentina must comply with the requirements of the National Administration of Medicines, Foods and Medical Technology – ANMAT.
Medical products must be registered by a company located in Argentina and authorized by ANMAT as Importer of Medical Products. According to Argentine regulations, it is preferable for registration that the manufacturer has a Free Sale Certificate from a country with high sanitary surveillance. Otherwise, ANMAT must inspect the manufacturer’s plant at the source.
We offer the following services in Argentina:
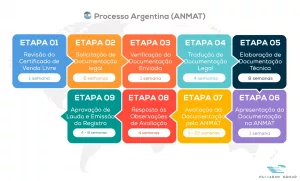
See below our complete ANMAT process flow
Passarini Group is a company that has the best specialists in Regulatory Consultancy of Medical Products, applying the One Stop Shop concept daily in your company with practical solutions.


