Service Regulation in Chile
The current Chilean regulation for medical devices focuses its work on the National Agency for Medical Devices, Innovation and Development (ANDID), one of the departments of the Chilean Institute of Public Health (ISPCH). The ANDID agency was born in November 2020, with one of its central objectives being to ensure the safety and performance of medical devices used in the country.
The current regulatory model establishes that there are 9 medical devices for which health registration with the ISPCH is required, which will allow their distribution, commercialization and/or use.
We offer the following services for medical devices in Chile:
Each of these medical devices has been incorporated into the health control regime in accordance with the provisions of the indicated exempt decrees, which also provide information on their risk classification. For their registration, each one must comply with the compliance verification, in which quality parameters are ensured that allow the commercialization of safe and effective products.
Current national regulations establish that, in order to obtain the sanitary registration of the MD, a conformity verification certification must be previously obtained, granted by an entity accredited by the National Institute of Standardization (INN) and authorized by the ISP.
Our team offers a wide range of services in Regulatory Affairs
See below our complete ISPCH process flow
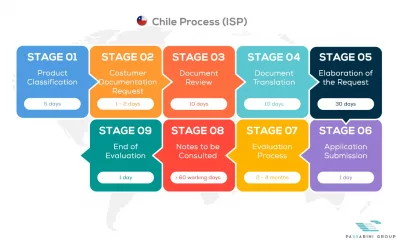
Passarini Group is a company that has the best specialists in Regulatory Consultancy of Medical Products, applying the One Stop Shop concept daily in your company with practical solutions.


