Regulation in Ecuador and more Services
Manufacturers of medical devices (dental products, biomedical equipment, biochemical and diagnostic reagents) who wish to distribute and market their products in Ecuador must comply with Ecuadorian legislation, that is, for a medical device to enter the country, it must obtain the health registration issued by the health authority (National Agency for Sanitary Regulation, Control and Surveillance – ARCSA), which is a unit of the Ministry of Public Health (MSP).
Passarini Group, through its technical and legal team in Ecuador, offers the comprehensive advice and support you need to comply with the regulatory framework in all its stages.
In Ecuador we have the following services:
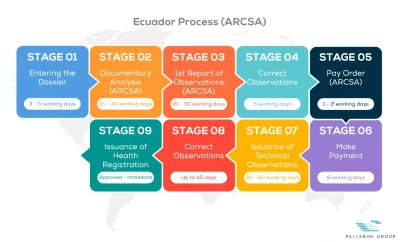
See below our complete ARCSA process flow
Passarini Group is a company that has the best specialists in Regulatory Consultancy of Medical Products, applying the One Stop Shop concept daily in your company with practical solutions.