Service Regulation in Paraguay
We are a company that provides regulatory affairs services to companies that manufacture Medical Devices, piercing-cutting materials and personal protective equipment classes I, II, III and IV, providing each client with the necessary information for registering products according to their classification before the National Directorate of Health Surveillance (DNVS).
To carry out all the work, we have a work team composed of trained professionals in the area of conducting.
Our team offers a wide range of Regulatory Affairs services:
See below our complete DINAVISA process flow
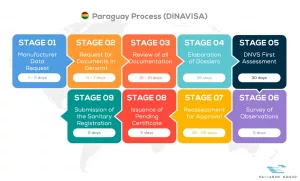
Passarini Group is a company that has the best specialists in Regulatory Consultancy of Medical Products, applying the One Stop Shop concept daily in your company with practical solutions.


