Consulting services for Peru and more information
Medical device manufacturers seeking market authorization in Peru must register with the Dirección General de Medicamentos, Drogas e Insumos (DIGEMID), a unit of the Peruvian Ministry of Health, unless the device type is exempt from registration. A Certificate of Free Sale (CFS) from the country of origin, country from which the devices are imported, or another recognized country is required for registration in Peru.
Passarini Group assist you in evaluating the Peruvian medical device regulatory framework as it applies to your device(s).
See below some of the services we can offer you for the Peruvian market:
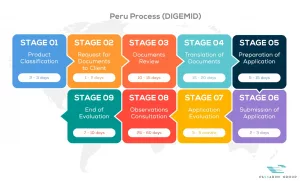
View our full DIGEMID process flow below
Passarini Group is a company that has the best specialists in Regulatory Consulting for Medical Products, applying the One Stop Shop concept daily in your company with practical solutions.
Please contact us for more information about our Regulatory Strategy Report for Peru.


