UDI Implementation Deadline for Class I Devices – FDA 510(k) Exempt!
We finally reach the UDI (Unique Device Identification) implementation deadline at the Food and Drug Administration (FDA) for class I devices – 510(k) exempt.
After the extension given by the FDA in July of this year, moving the deadline from September 24, 2022 to December 8, 2022 (adding 75 calendar days), there are no more loopholes for registrations of class I medical devices with the FDA, without the implementation of Unique Device Identification.
Now, all products will need to have Unique Device Identification to be FDA compliant. It is the first regulatory agency to adopt UDI for all device classes.
For Europe (CE Certification – MDR 2017/745), there are still open deadlines depending on the risk class of the medical product and for ANVISA, we already have deadlines established since the end of last year (RESOLUTION – RDC Nº 591, OF 21 DECEMBER 2021 – resolution that entered into force from January 10, 2022).
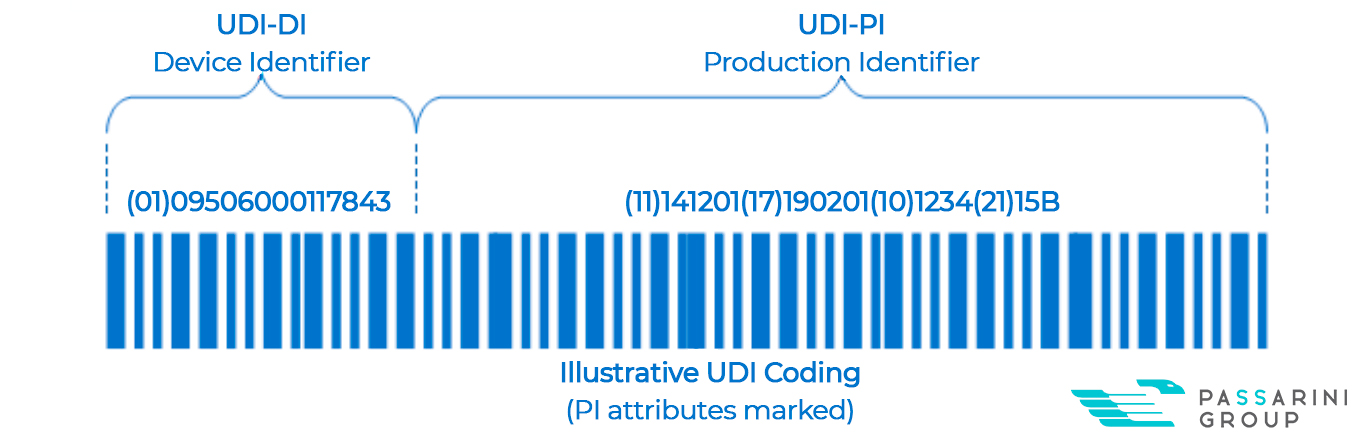
The Unique Device Identification is composed of two groups of codes (UDI = DI + PI) and must be presented in two forms: MR (Machine Readable – barcode or QR Code) and HR (Human Readable – numbers).
The DI (Device Identifier) will be the numbers that will identify the manufacturer/labeler and model of the device. They will be fixed by each device model and have the identification 01 in parentheses – (01) as a prefix.
The PI (Production Identifier) will be the numbers that will identify production information such as expiration date, manufacturing date, lot number, and serial number. They will be variable numbers and have as a prefix, the identifications between parentheses 17, 11, 10, and 21. (17) “Expiration date”, (11) “Manufacturing date”, (10) “Batch number” and (21) “Serial number”.
All these codes are acquired through an issuing agency and must be registered on parallel platforms.
For further clarification, please contact us. Our team is available!
For more information and explanations about it, send us an e-mail: contato@passarini.com.br
Passarini Group
Published: 15/12/2022


