Passarini Blog
What are your challenges for 2019?
Start 2019 with an exclusive gift from Passarini Regulatory Affairs Are your goals and challenges for 2019 already set? How was it for you and your company to plan the year? Passarini Regulatory Affairs is here to offer you the best solutions in the healthcare [...]
Dubai 2019
Arab Health 2019 Check out the videos below for news from Dubai. Choose the language of the video and click the link below: Passarini Regulatory Affairs has been working for more than 10 years with ANVISA and has highly knowledgeable professionals [...]
Events 2019
Events 2019 Speak with our consultants during the Fairs. For more information send us an e-mail: contato@passarini.com.br Passarini Regulatory Affairs Published: 10/01/2019 Mais notícias:
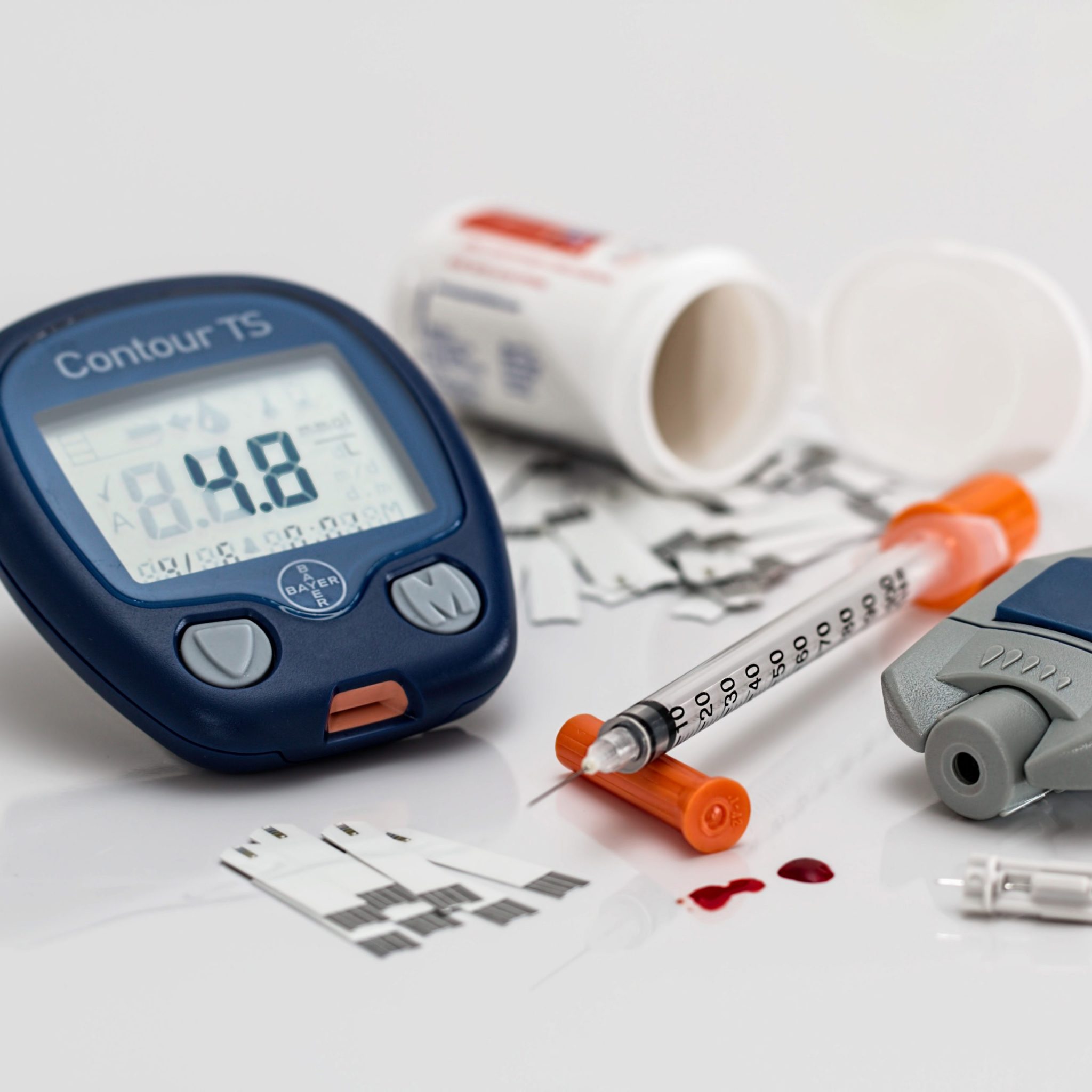
Health Product Labeling Information
Health Product Labeling Information There has been increasing demand for questions about the correct interpretation of the label content of single-use products available on the market. For a better understanding about it, ANVISA published on October 3, 2013, a technical note clarifying and detailing the [...]
Promo – August in Double
August in Double Check below the promotion that will stir your month of August. Get 02 ANVISA REGISTRATION PROCESSES and pay only one. Send you requeriments to contato@passarini.com.br and get your quotation. Rules: The second application must be done until December 14th, 2018 and must [...]
FIME 2018
FIME 2018 Passarini Regulatory Affairs confirms presence of its consultants at FIME 2018 - medical fair in Orlando - USA. July 17-19, 2018 Location: Orange County Convention Center, Orlando - Florida Schedule a meeting by email: contact.usa@passarini.com.br Learn more about the event: https://www.fimeshow.com/en/home.html Keep [...]